The mass of one mole of is. How to find mass from moles and molar.
Hence number of moles in calcium is 2.

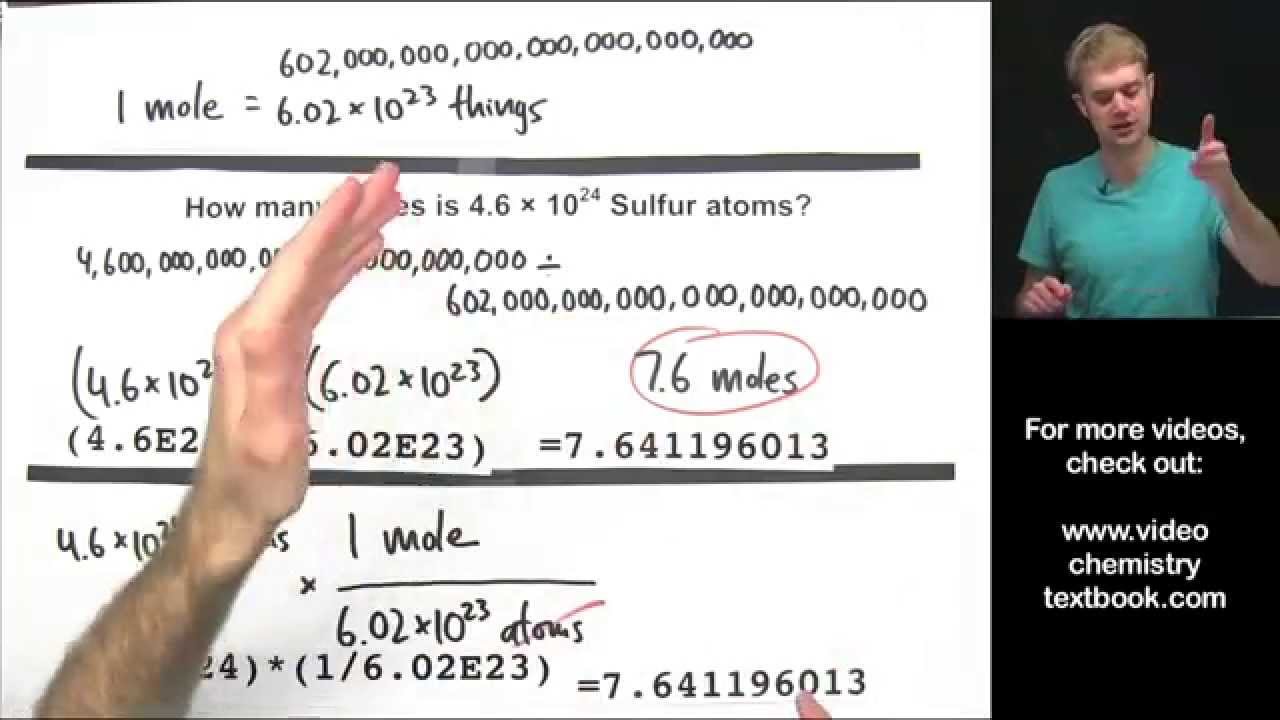
. In chemistry a mole is a really big numberThis number 602 x 10 23 comes from the number of atoms in 12 g of carbon-12 this is the carbon isotope with six protons and. Since 1 mole 6022140710 23 particles atoms molecules ions etc 1 joule per mole is equal to 1 joule divided by 60221407610 23 particles 16605410 24 joule per. To Calculate Number of Molecules in a Given mole.
First convert the grams to moles using the molar mass and then use Avogadros number to find. Calculate the number of moles of carbon dioxide molecules in 22 g of CO 2. A mole of a substance or a mole of particles is defined as exactly 60221407610²³ particles which may be atoms molecules ions or electrons.
A r relative atomic mass of C 12 A r of O 16. Finding the number of moles Question. Calculate the number of moles and number of molecules of following.
Thus the known information is. Find out the number of moles in 0325 grams of barium hydroxide. We know the atomic mass of calcium is 40.
Use the ideal gas law to calculate the number of moles of the sample. The mole is important because it allows. Mass of 0325 gram.
Formula to calculate number of moles weightatomic mass of the element. 0032 mg of methane. N is the number of moles mol m is the mass in grams g M is the molar mass gmol We can understand it better by solving some examples.
To figure this out you will need the molar mass of NaCl which is 5844 gmol. Dividing both sides by eqRT eq causes this to cancel from the right side.

Mole Ratios Mole Ratio Relationship

Molarity M The Concentration Of A Solution As The Number Of Moles Of Solute Chemistry Education Biology Notes Chemistry

How To Find The Mole Ratio And Molar Mass Youtube High School Chemistry Chemistry Molar Mass
Converting Between Moles Atoms And Molecules Teaching Chemistry Chemistry Textbook High School Chemistry

0 Comments